Difference between revisions of "Cucurbita okeechobeensis"
Gentes Herb. 2: 179. 1930.
FNA>Volume Importer |
FNA>Volume Importer |
||
| Line 11: | Line 11: | ||
|label=Conservation concern | |label=Conservation concern | ||
}} | }} | ||
| − | |basionyms={{Treatment/ID/ | + | |basionyms={{Treatment/ID/Basionym |
|name=Pepo okeechobeensis | |name=Pepo okeechobeensis | ||
|authority=Small | |authority=Small | ||
| + | |publication_title=J. New York Bot. Gard. | ||
| + | |publication_place=31: 12, figs. 1, 2. 1930 | ||
}} | }} | ||
|synonyms= | |synonyms= | ||
| Line 29: | Line 31: | ||
|discussion=<p>Subspecies 2 (1 in the flora).</p><!-- | |discussion=<p>Subspecies 2 (1 in the flora).</p><!-- | ||
--><p><i>Cucurbita okeechobeensis</i> originally was common in extensive pond-apple (or custard-apple, <i>Annona glabra</i>) forests on the southern shore of Lake Okeechobee (J. K. Small 1922). By the time it was described in 1930, most of these forests had been destroyed. Currently <i>C. okeechobeensis</i> is known only in two population systems Decline and current rarity of <i>Cucurbita okeechobeensis</i> has resulted mostly from conversion of swamp forests to agriculture and from water-level management in Lake Okeechobee. Fluctuations in lake level are necessary as high levels facilitate dispersal, and the inundation and withdrawal create open habitats for quick growth of the seedlings. Exotic vegetation around the edges of the lake, especially Melaleuca, also is a threat.</p><!-- | --><p><i>Cucurbita okeechobeensis</i> originally was common in extensive pond-apple (or custard-apple, <i>Annona glabra</i>) forests on the southern shore of Lake Okeechobee (J. K. Small 1922). By the time it was described in 1930, most of these forests had been destroyed. Currently <i>C. okeechobeensis</i> is known only in two population systems Decline and current rarity of <i>Cucurbita okeechobeensis</i> has resulted mostly from conversion of swamp forests to agriculture and from water-level management in Lake Okeechobee. Fluctuations in lake level are necessary as high levels facilitate dispersal, and the inundation and withdrawal create open habitats for quick growth of the seedlings. Exotic vegetation around the edges of the lake, especially Melaleuca, also is a threat.</p><!-- | ||
| − | --><p><i>Cucurbita okeechobeensis</i> subsp. martinezii (L. H. Bailey) Andres & Nabhan ex T. Walters & D. S. Decker (C. martinezii L. H. Bailey) is widespread in a narrow strip of eastern Mexico (Chiapas, Oaxaca, Puebla, San Luis Potosí, Tamaulipas, Veracruz), often in riparian vegetation and as a weed in coffee and citrus plantations. Subspecies okeechobeensis is morphologically distinct only in its densely pubescent staminate hypanthia and pubescent pistillate ovaries. There is no sterility barrier between the two, and F1 hybrids are fertile (R. W. Robinson and J. T. Puchalski 1980). There is considerable allelic variation within subsp. martinezii (T. C. Andres and G. P. Nabhan 1988) but little or none in <i></i>subsp.<i> okeechobeensis</i>.</p><!-- | + | --><p><i>Cucurbita okeechobeensis</i> subsp. martinezii (L. H. Bailey) Andres & Nabhan ex T. Walters & D. S. Decker (C. martinezii L. H. Bailey) is widespread in a narrow strip of eastern Mexico (Chiapas, Oaxaca, Puebla, San Luis Potosí, Tamaulipas, Veracruz), often in riparian vegetation and as a weed in coffee and citrus plantations. Subspecies okeechobeensis is morphologically distinct only in its densely pubescent staminate hypanthia and pubescent pistillate ovaries. There is no sterility barrier between the two, and F1 hybrids are fertile (R. W. Robinson and J. T. Puchalski 1980). There is considerable allelic variation within subsp. martinezii (T. C. Andres and G. P. Nabhan 1988) but little or none in <i></i></i>subsp.<i><i> okeechobeensis</i>.</p><!-- |
--><p>R. W. Robinson and J. T. Puchalski (1980) found through isozyme analysis a single allelic difference between the Okeechobee and Martinez gourds. T. W. Walters and D. S. Decker (1991) confirmed the minor allelic difference and estimated the time of divergence as</p><!-- | --><p>R. W. Robinson and J. T. Puchalski (1980) found through isozyme analysis a single allelic difference between the Okeechobee and Martinez gourds. T. W. Walters and D. S. Decker (1991) confirmed the minor allelic difference and estimated the time of divergence as</p><!-- | ||
--><p>about 450,000 years, with the conclusion that the two were appropriately treated as conspecific. A population of <i>Cucurbita okeechobeensis</i> was recently discovered in the Dominican Republic (B. Peguero and F. Jiménez 2005); the authors were unable to assign the plants to either subspecies and it is unclear whether the population is natural or the result of introduction within the last century (M. Nee, pers. comm.).</p><!-- | --><p>about 450,000 years, with the conclusion that the two were appropriately treated as conspecific. A population of <i>Cucurbita okeechobeensis</i> was recently discovered in the Dominican Republic (B. Peguero and F. Jiménez 2005); the authors were unable to assign the plants to either subspecies and it is unclear whether the population is natural or the result of introduction within the last century (M. Nee, pers. comm.).</p><!-- | ||
| Line 56: | Line 58: | ||
|publication year=1930 | |publication year=1930 | ||
|special status=Conservation concern | |special status=Conservation concern | ||
| − | |source xml=https://jpend@bitbucket.org/aafc-mbb/fna-data-curation.git/src/ | + | |source xml=https://jpend@bitbucket.org/aafc-mbb/fna-data-curation.git/src/f6b125a955440c0872999024f038d74684f65921/coarse_grained_fna_xml/V6/V6_82.xml |
|genus=Cucurbita | |genus=Cucurbita | ||
|species=Cucurbita okeechobeensis | |species=Cucurbita okeechobeensis | ||
Revision as of 21:17, 24 September 2019
Distribution
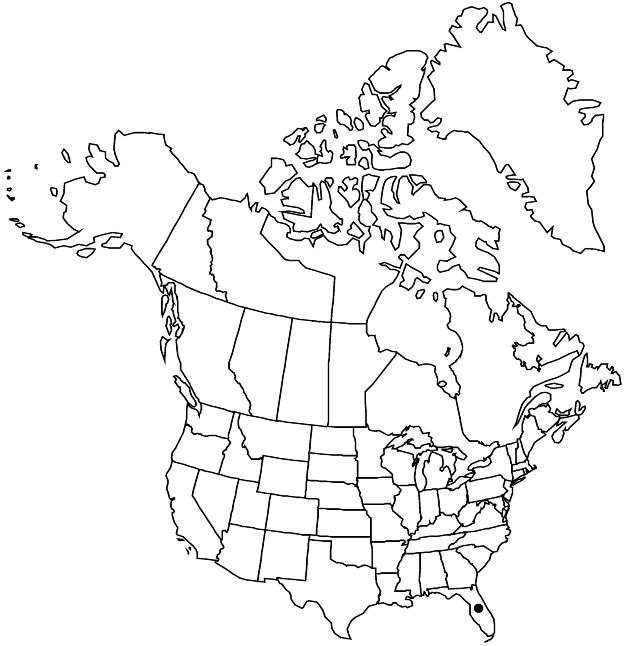
United States, Mexico, West Indies (Hispaniola).
Discussion
Subspecies 2 (1 in the flora).
Cucurbita okeechobeensis originally was common in extensive pond-apple (or custard-apple, Annona glabra) forests on the southern shore of Lake Okeechobee (J. K. Small 1922). By the time it was described in 1930, most of these forests had been destroyed. Currently C. okeechobeensis is known only in two population systems Decline and current rarity of Cucurbita okeechobeensis has resulted mostly from conversion of swamp forests to agriculture and from water-level management in Lake Okeechobee. Fluctuations in lake level are necessary as high levels facilitate dispersal, and the inundation and withdrawal create open habitats for quick growth of the seedlings. Exotic vegetation around the edges of the lake, especially Melaleuca, also is a threat.
Cucurbita okeechobeensis subsp. martinezii (L. H. Bailey) Andres & Nabhan ex T. Walters & D. S. Decker (C. martinezii L. H. Bailey) is widespread in a narrow strip of eastern Mexico (Chiapas, Oaxaca, Puebla, San Luis Potosí, Tamaulipas, Veracruz), often in riparian vegetation and as a weed in coffee and citrus plantations. Subspecies okeechobeensis is morphologically distinct only in its densely pubescent staminate hypanthia and pubescent pistillate ovaries. There is no sterility barrier between the two, and F1 hybrids are fertile (R. W. Robinson and J. T. Puchalski 1980). There is considerable allelic variation within subsp. martinezii (T. C. Andres and G. P. Nabhan 1988) but little or none in subsp. okeechobeensis.
R. W. Robinson and J. T. Puchalski (1980) found through isozyme analysis a single allelic difference between the Okeechobee and Martinez gourds. T. W. Walters and D. S. Decker (1991) confirmed the minor allelic difference and estimated the time of divergence as
about 450,000 years, with the conclusion that the two were appropriately treated as conspecific. A population of Cucurbita okeechobeensis was recently discovered in the Dominican Republic (B. Peguero and F. Jiménez 2005); the authors were unable to assign the plants to either subspecies and it is unclear whether the population is natural or the result of introduction within the last century (M. Nee, pers. comm.).
The closest relative of Cucurbita okeechobeensis and C. martinezii appears to be C. lundelliana L. H. Bailey (T. C. Andres and G. P. Nabhan 1988), which is geographically isolated in the Yucatán Peninsula but can form artificial hybrids with them. Cucurbita lundelliana differs in its orange corollas, less lignified exocarp, and seeds with crenulate margins.